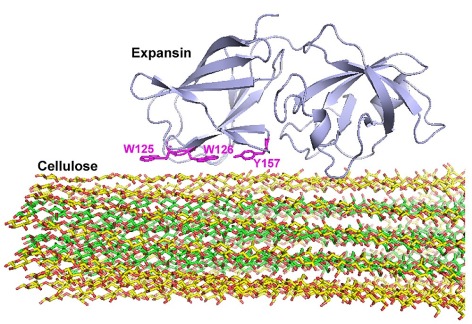
This illustration shows the parts of the expansin protein (magenta) that bind to the surface of specific regions of plant cell walls. Larger image. Illustration courtesy of Mei Hong.
AMES, Iowa – Using a new and super-sensitive instrument, researchers have discovered where a protein binds to plant cell walls, a process that loosens the cell walls and makes it possible for plants to grow.
Researchers say the discovery could one day lead to bigger harvests of biomass for renewable energy.
Finding that binding target has been a major challenge for structural biologists. That’s because there are only tiny amounts of the protein involved in cell growth and because cell walls are very complex, said Mei Hong, one of the project’s lead researchers who’s an Iowa State University professor of chemistry and a faculty scientist with the U.S. Department of Energy’s Ames Laboratory.
A paper describing the discovery, “Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls,” was just published by the Proceedings of the National Academy of Sciences Online Early Edition. Hong and Daniel Cosgrove, professor and holder of the Eberly Chair in Biology at Penn State University, are the lead authors.
The research team also includes Tuo Wang, an Iowa State graduate student in chemistry and a graduate assistant for the Ames Laboratory; Linghao Zhong, an associate professor of chemistry at Penn State Mont Alto; Yong Bum Park, a post-doctoral scholar in biology at Penn State; plus Marc Caporini and Melanie Rosay of the Bruker BioSpin Corp. in Billerica, Mass.
Three grants from the U.S. Department of Energy supported the research project.
Iowa State’s Hong has long used solid-state nuclear magnetic resonance (NMR) spectroscopy to study structural biology, including the mechanism used by the flu virus to infect host cells. But in this case, that technology wasn’t sensitive enough to identify the binding site of the expansin protein.
So the researchers – working with specialists from the Bruker BioSpin Corp., a manufacturer of scientific instruments – used a technology called dynamic nuclear polarization (DNP), to enhance the sensitivity of spectroscopy instruments. The technology was developed by Robert Griffin, a professor of chemistry at the Massachusetts Institute of Technology.
The researchers studied Arabidopsis thaliana, often used as a model subject in plant science studies, and found the protein binds to specific regions of cellulose microfibrils, the long, parallel chains of cellulose that make up plant cell walls. The action weakens the network formed by a cell wall’s cellulose, hemicellulose and pectins, loosening the cell wall and allowing cell growth.
The researchers found the target site is the part of the cellulose microfibril that is enriched with the hemicellulose xyloglucan. The target site has a different cellulose structure than a plant’s bulk cellulose.
“This result wasn’t trivial to get and we are quite happy that the DNP NMR technology is so useful for understanding this plant biochemistry question,” Hong said.
And yes, she said, “Our result could be exploited for practical benefits.” Knowing where expansin binds to cell walls “might help biochemists design more potent expansins to loosen the cell wall and stimulate plant growth and thus better harvest bioenergy.”
